Imagine an Olympic final of tug-of-war where both teams are evenly matched, the rope would stay in the middle. In the final moments, Team A substitutes on their strongest player causing the rope to shift in their favour. That is, until Team B comes up with new strategies to counteract Team A’s newfound strength and re-establish the balance.
As you read this article on equilibrium and acid reactions, the relevance of this analogy will become clearer. HSC Chemistry Module 5 centres around the concept of balance. When the rope is centred, the system is in “equilibrium” (more on this later).
Getting a grip on the core concepts in HSC Chemistry Module 5 will be immensely helpful as you progress through the course. So without further ado, let’s discuss some of the key concepts and skills you need to know, to get a Band 6 in this subject!
Understanding chemical equilibrium
Reversible reactions
Some chemical reactions are “reversible”. That is, reactants are converted into products, and reactants can be reformed from products, simultaneously. Such reactions are denoted by a double-headed arrow (⇌) in the chemical equation.
As you may have guessed, irreversible reactions describe chemical reactions where products cannot reform into reactants. These reactions are denoted by a single-headed arrow (→), representing the forward reaction only.
Static equilibrium
When reactions are irreversible, the system will reach a state of static equilibrium. This means the reaction goes to completion, after which it does not proceed in the forward or reverse reaction anymore.
Dynamic equilibrium
Reversible reactions can reach a state of dynamic equilibrium, where the rate of the forward reaction and reverse reaction are equal and non-zero. As there is no net change in the concentration of reactants or products, the system APPEARS static at a macroscopic level. However, the system is not in static equilibrium because microscopically, both reactions occur continuously and balance each other out.
Note: All reactions in dynamic equilibrium, must be reversible (by definition). However, not all reversible reactions are always in a state of dynamic equilibrium!
Linking back to our earlier analogy of tug-of-war, the scenario can be described as a dynamic equilibrium. On a large scale, the rope is not moving (i.e., appearing static). However, on a smaller scale, both Teams A and B (i.e., the reactants and products) are continuously exerting equal force.



Image from Vecteezy.
Le Chatelier’s Principle
If there’s one thing to remember from this article, it’s Le Chatelier’s Principle (or LCP for short).
Le Chatelier’s Principle states that: when a closed system at dynamic equilibrium is disturbed, it shifts to minimise the impact of the disturbance until a new equilibrium is established.
Many factors affect equilibrium. These disturbances and can include dilution, changing temperature, pressure, volume, or the addition or removal of a species. LCP helps predict the direction in which a system will shift in response to these changes.
We can link this concept back to the earlier analogy again. The disturbance - Team A adding their strongest player—parallels an increase in the concentration of reactants. Team B’s counter-strategy symbolises a response from the chemical system to restore dynamic equilibrium, just like how the reaction adjusts by decreasing reactant concentration or increasing product concentration to balance the changes.
Note: to do well in HSC Chemistry Module 5 questions, always define LCP before applying it in your short answer responses!
An example of Le Chatelier’s principle and dynamic equilibrium
Consider this gaseous system involving nitrogen dioxide and dinitrogen tetroxide, which are brown and colourless, respectively:
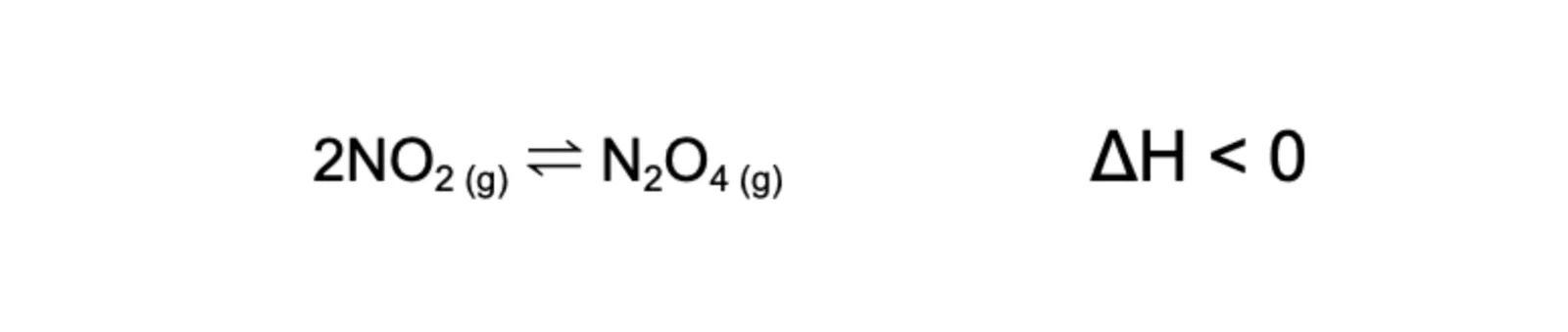
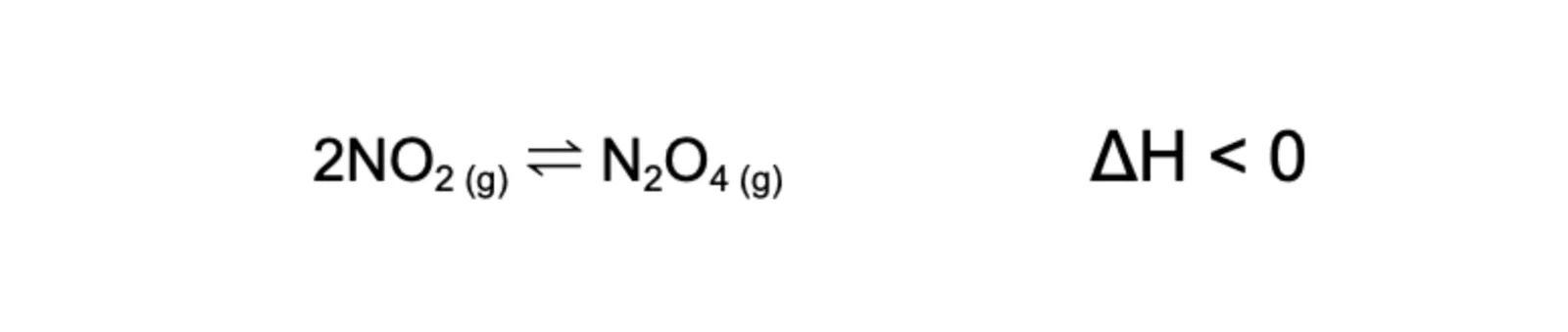
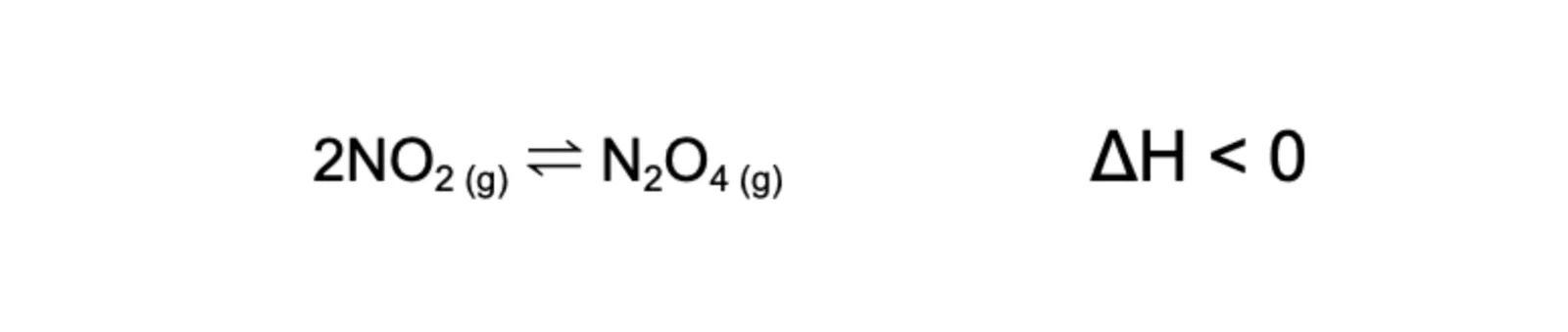
When the system’s volume decreases, pressure increases (by Boyle’s Law). According to LCP, the system shifts toward the side with fewer gas moles to decrease pressure. Thus, it shifts right (2:1 gas molar ratio), favouring the forward reaction to produce more dinitrogen tetroxide, making the system appear more brown.
Collision theory
The above phenomenon can also be described using collision theory, which states that chemical reactions occur due to successful collision, in which particles must collide in the correct orientation and with sufficient energy to overcome activation energy.
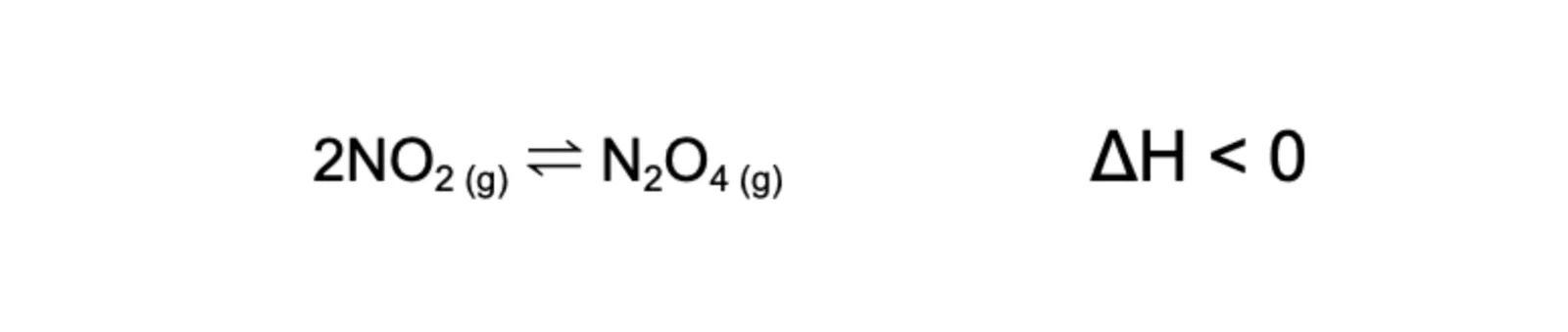
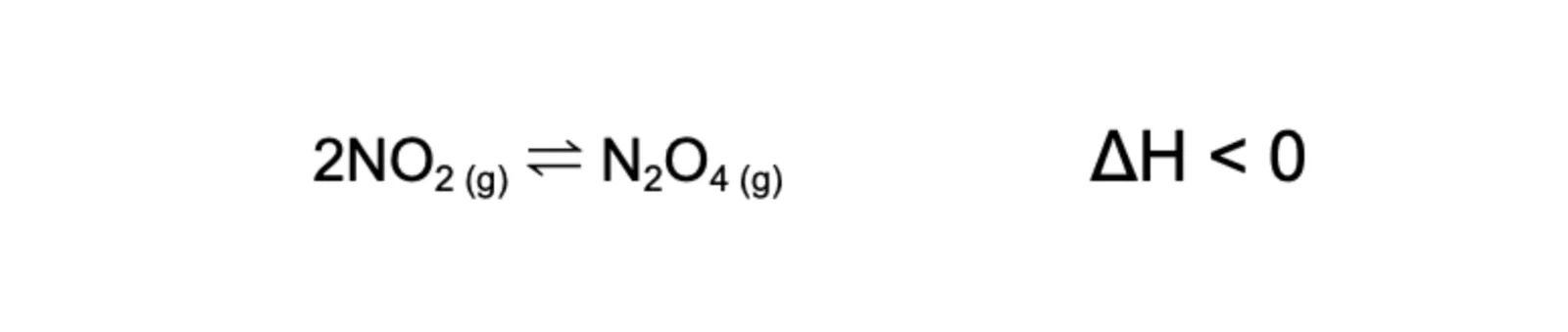
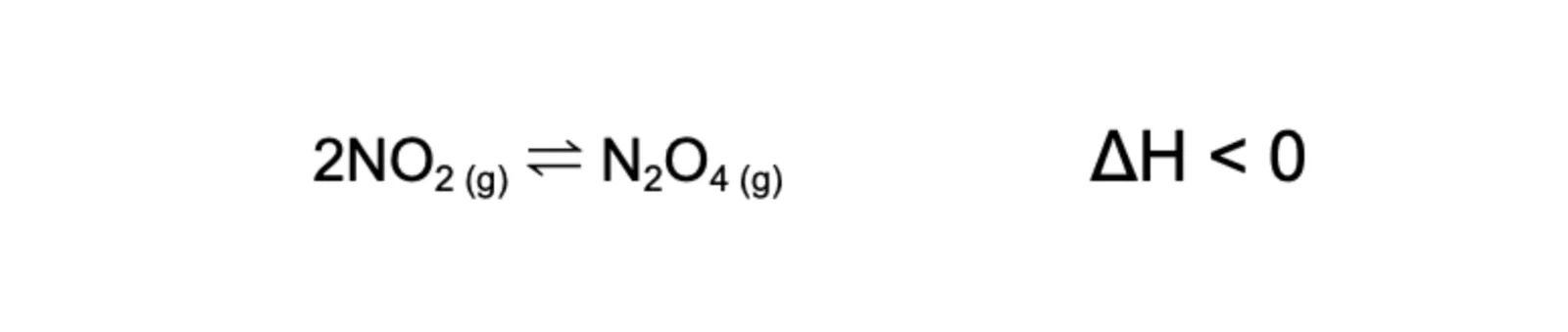
Using the same example above, when volume is decreased/pressure is increased, all particles come in close proximity. As there are more reactant particles than product particles, the forward direction is affected more resulting in a shift towards the right.
Challenge: Predict what happens when the system is warmed! Try using LCP, and check your answer at the article’s end. For an added challenge, think about it in terms of collision theory. Solutions will be at the end of this article!
Interpreting concentration, pressure, and temperature disturbances on graphs
A common way to represent disturbances to an equilibrium system is through concentration-time graphs, where each change has a distinct pattern. The graph will also show a favouring of the forward reaction or reverse reaction, to minimise the disturbance (by LCP). To ace equilibrium and acid reactions, it is crucial to get comfortable interpreting these graphs.
Here’s a brief guide for each disturbance:
-
Concentration: Adding or removing a species causes an immediate sharp increase or decrease in its concentration. The system shifts toward the removed species or away from the added one.
-
Volume/Pressure: Changing volume alters pressure inversely, causing a sharp concentration change in all species. The system shifts toward more gas moles if pressure decreases or fewer gas moles if pressure increases.
-
Temperature: Heating or cooling causes a gradual shift without immediate concentration changes. If the system is heated, it will shift in the endothermic direction while if it is cooled, it will shift in the exothermic direction.
Rate-time graphs are also commonly assessed, as seen in the example below.
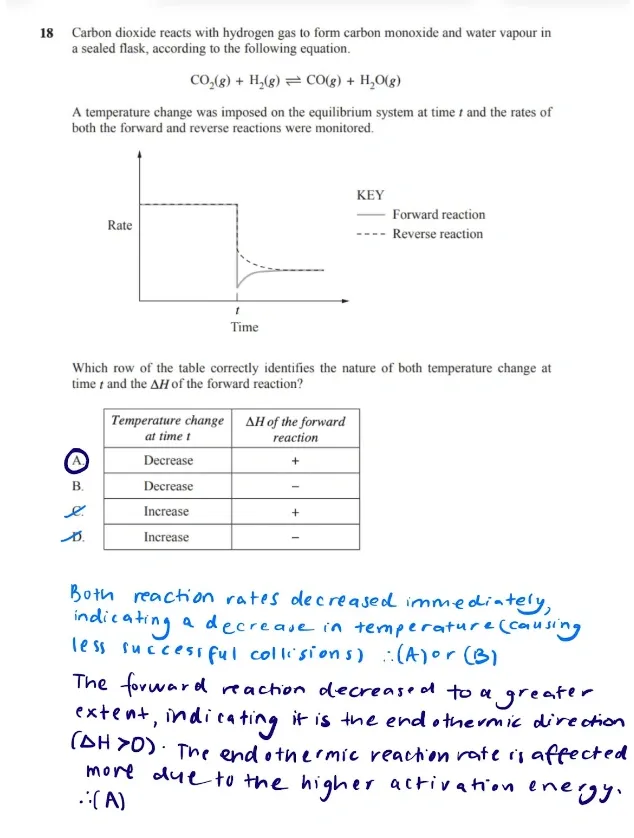


The best way to get good at these, is with past paper questions available from your school or online.
PS: Project Academy’s HSC Chemistry course gives you access to a huge range of resources across their in-house apps, that you can use to practise your graph-reading skills.
Calculating the equilibrium constant
What is the equilibrium constant?
The equilibrium constant is a ratio of the equilibrium concentrations of products over the reactants raised to their coefficients. Or:
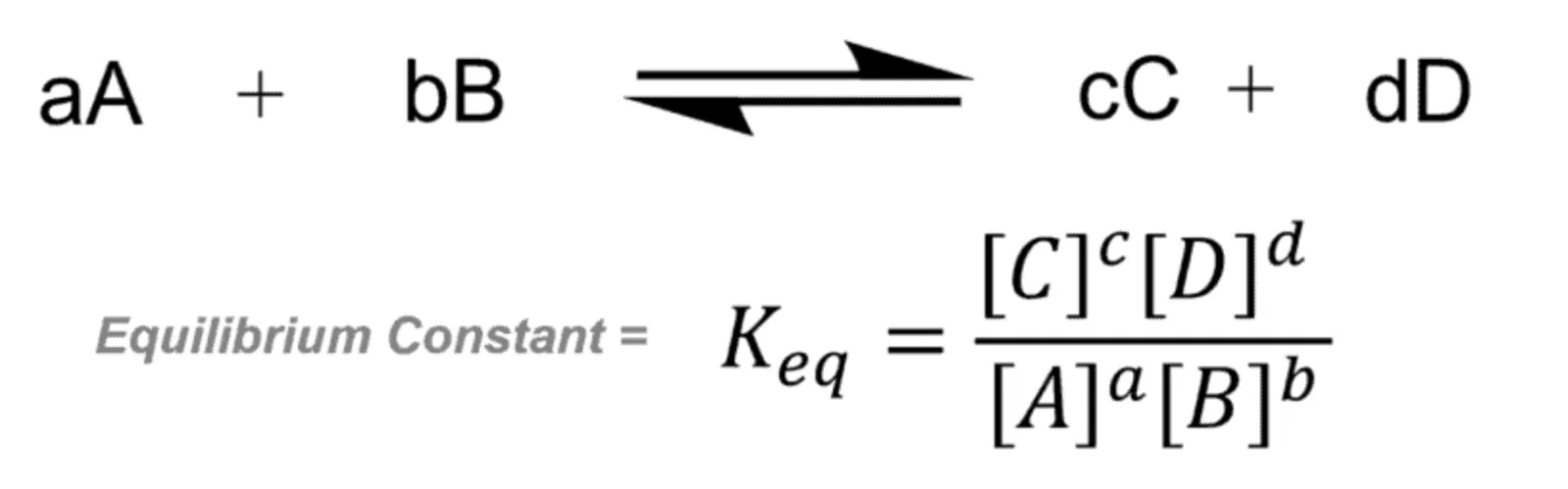


It can be notated as Keq, Kc, or just K.
The equilibrium constant tells us the equilibrium position of a reversible reaction, by qualitatively analyse the relative amounts of reactants and products. Loosely speaking,
-
A large equilibrium constant means [products] > [reactants]
-
A small equilibrium constant means [reactants] > [products]
Other key things to note about Keq include:
-
Keq does not have a unit (e.g. cm, m etc.)
-
Keq is constant, for a constant temperature - i.e., the only disturbance that can change the value of Keq is temperature.
-
Pure solids and liquids are not included in the calculation (since their concentrations wouldn’t change)
How do we calculate the equilibrium constant?
When doing questions related to equilibrium and acid reactions, you’ll often be asked to find Keq. The question will typically provide non-equilibrium concentrations, and you have to find the equilibrium concentrations, to then find Keq. One way of doing this, is to use an ICE table.
-
I = Initial concentrations of reactants and products
-
C = Change in concentrations to reach equilibrium. This involves matching with the molar ratios of the balanced chemical equation.
-
E = Equilibrium concentration of reactants and products
To effortlessly find Keq each time, simply:
-
Write down the equilibrium constant expression.
-
Fill in an ICE table using the given information in the question.
-
Find the equilibrium concentration of reactants and products in the table.
-
Calculate K.
Equilibrium constant vs. Reaction quotient
If you substitute concentrations into the K expression when the system is not in equilibrium, you will instead get the “reaction constant”, or Q. Q simply describes the relative amounts of reactants and products when NOT in equilibrium.
Using the reaction quotient to predict shift in an equilibrium
Using Le Chatelier’s Principle, we know that a system that is not in equilibrium due to disturbance, will shift to be in equilibrium. Therefore, we know Q will tend towards Keq. In other words:
-
If Q < Keq, the forward reaction (i.e. right) will be favoured, to form more product and increase Q
-
If Q > Keq, the reverse reaction (i.e. left) will be favoured, to form more reactant and decrease Q.
-
If Q = Keq, the system is in dynamic equilibrium already.
Combining all of the theory above, we can tackle a tricky exam question. For example:



Understanding solubility equilibria
Dissolution of an ionic compound or salt
Other examples of reversible reactions are the dissolution and precipitation of an ionic salt in solution, such as sodium chloride (e.g. NaCl). In the process of dissolution, the partially negative oxygen in water is attracted to the cation (e.g. sodium ions or Na+) while the partially positive hydrogen in water is attracted to the anion (e.g. chloride ions or Cl-). Thus, the ions form ion dipole forces with water, which is an exothermic process. However, the ionic bonds between ions in the lattice and the hydrogen bonds between water molecules are also broken in the reaction, which is an endothermic process. Overall, the dissolution of a salt can be either exothermic or endothermic depending on whether the energy consumed by bond breaking outweighs the energy released from bond formation.
Eventually, the rate of dissolution and precipitation will be equal, meaning there is no change in the concentration of Na+ and Cl- ions, and thus the system is in dynamic equilibrium.
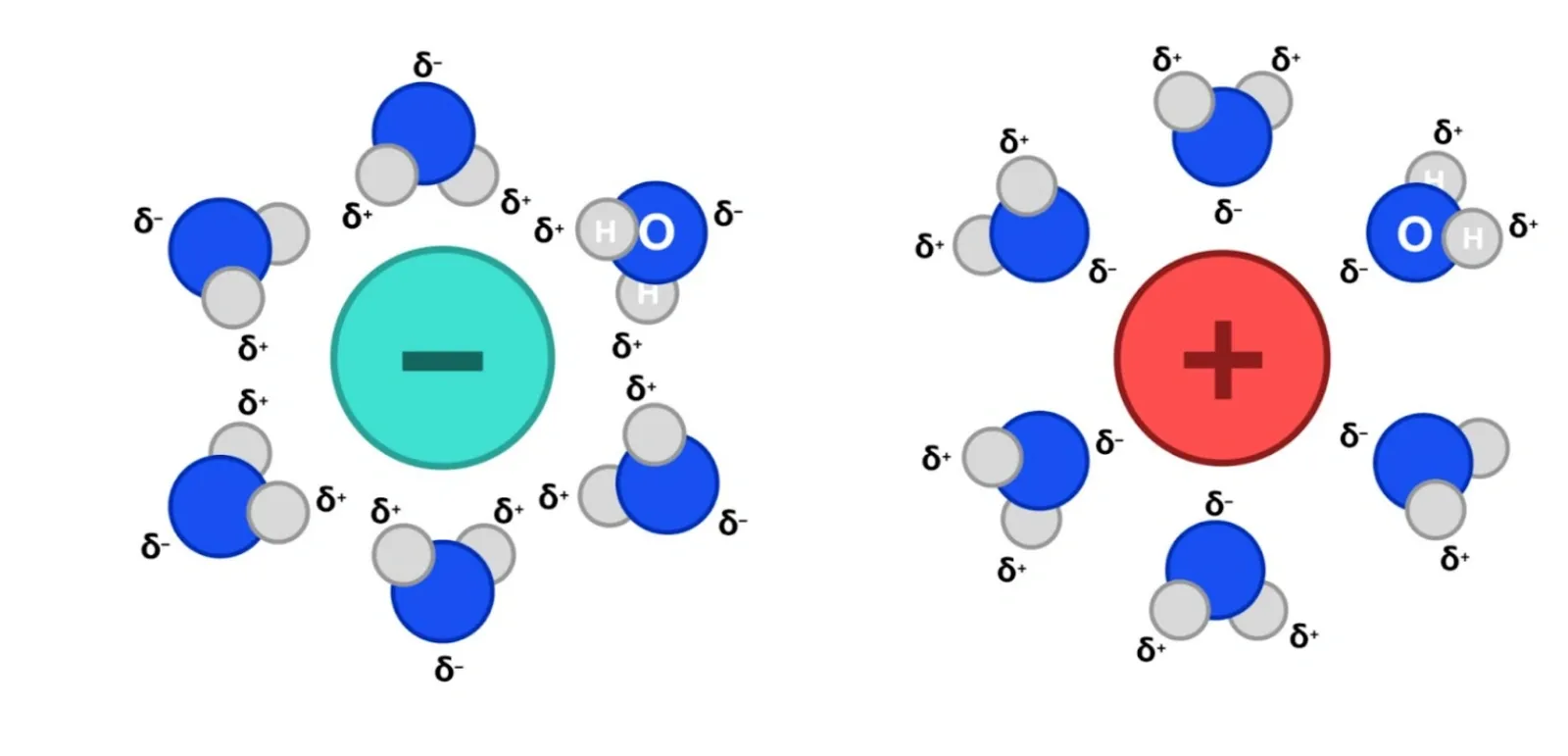


Explore more diagrams like this in Project Academy’s Books app, which offers lesson workbooks, video tutorials, worked solutions, textbook-like explanations, and more!
What is solubility equilibria and Ksp?
When the above happens, the solution has become perfectly saturated and in dynamic equilibrium. For solubility equilibria, we calculate a special equilibrium constant called the solubility product (Ksp). When calculating Ksp, use the same formula as Keq. Similarly, Qsp is the reaction quotient for solubility equilibria. The Ksp values appear on the first page of the data sheet.
Ksp can be used to:
-
Find the molar solubility of solutions to compare their ability to dissolve
-
Compare against Qsp, to predict whether a precipitate will form
Using Ksp to predict solubililty
The Ksp values appear on the first page of the data sheet. Ksp can be used to find the molar solubility of solutions to compare their ability to dissolve. Moreover, Qsp can be compared to Ksp to predict whether a precipitate will form.
There are three types of solutions: unsaturated, saturated, and supersaturated.
-
Unsaturated solution: Qsp < Ksp and a precipitate will not form as it contains less than the maximum amount of solute able to be dissolved
-
Saturated solution: cannot dissolve any more solute when added resulting in the formation of a precipitate as Qsp > Ksp.
-
Supersaturated solution: created when a saturated solution is heated and then rapidly cooled, allowing more than the maximum amount of solute to dissolve, resulting in the formation of crystals.
Conclusion
We hope this article has help you understand the key concepts and skills required to ace Module 5 of HSC Chemistry. If this content interests you and you are about to start HSC Chemistry, come along to one of our FREE PROCON masterclasses during the upcoming school holidays to learn more about Module 5 and Project Academy.
*Answer to Challenge
LCP: When the system is heated, it shifts in the reverse endothermic direction. This results in more NO2 being produced and the system appearing less brown.
Collision Theory: When the system is heated, all the particles have more kinetic energy resulting in an increased number of successful collisions in the forward and reverse directions. However, as the reverse endothermic reaction has a higher activation energy than the forward exothermic reaction, a greater proportion of product particles will have sufficient energy for a successful collision following the temperature increase. As a result, the reverse endothermic reaction is favoured and the system shifts left to produce more NO2 causing the system to appear less brown. As products are converted to reactants, the reverse direction slows and the forward direction speeds up until the rates equalise and equilibrium is re-established.








