Have you ever wondered how soap can clean your hands? Or how plastics are made? Or how they get your favourite candy to taste like pineapples?
Welcome to this module 7 chemistry guide! Today we’re focused on Organic Chemistry, which is the study of organic compounds. Organic compounds have a variety of applications in real life, some of which I’ve hinted to above. You’ll learn everything, from their structure, to their physical and chemical properties, to the IUPAC nomenclature system, and much more.
The goal of this article is to break down key concepts taught within Module 7, as well as to emphasise the key skills that will help you score well in your HSC exam.
Let’s dive in :)
What are Organic Compounds?
Before we proceed, let’s clarify what they are. An organic compound is essentially one that is carbon-based (i.e. contains carbon atoms). They’re all around us, such as in fuels for cars, plastics, and also soaps! The simplest types (which are also the ones we will be focusing on) are hydrocarbons - compounds made of hydrogen and carbon atoms.
Key Concepts
Properties of Organic Compounds
In Module 7 we’ll be learning about all different classes of organic compounds, and one of the key concepts the syllabus emphasises is to be able to explain the properties within and between each group of compounds. To do this, we’ll be revisiting something introduced in year 11 chemistry - intermolecular and intramolecular forces!
Boiling Points of Organic Compounds
Boiling point has a direct relationship with the strength of intermolecular forces a compound experiences, i.e. the stronger the IMFs of a compound, the higher its boiling point. Hydrogen bonds are the strongest, followed by dipole-dipole interactions and finally dispersion forces.
Solubility of Organic Compounds
Next, we can also talk about solubility. For a compound to be soluble in water, it needs to experience strong enough intermolecular forces with water, to break apart the IMFs between its molecules, and between the water molecules. Generally, “like dissolves like” i.e. polar compounds will dissolve in polar solvents (such as water) whilst non-polar compounds will dissolve in non-polar solvents (such as cyclohexane).
Reactivity of Organic Compounds
Reactivity depends on the intramolecular forces in a molecule as chemical bonds must be broken and reformed for a reaction to occur. In organic chemistry, we need to look at the covalent bond strength, determined by the polarity of the bond, and whether any reactive pi-bonds are present.
Reactions of Organic Compounds
There are many reactions to learn when studying organic compounds. So, how should we approach this? We can break them down by the types of compounds involved helps to make it less daunting.
Here are the organic reactions you’ll be required to learn, predict the products of, and write equations for:
-
Hydrocarbons
-
Addition of H2/X2/HX/H2O to alkenes
-
Substitution of X2 with alkanes
-
-
Alcohols
-
Oxidation of primary and secondary alcohols
-
Dehydration
-
Substitution with HX
-
-
Other
-
Substitution of haloalkanes with a strong base
-
Fermentation
-
Combustion of hydrocarbons/alcohols
-
Esterification
-
When writing reaction equations for organic compounds, there are a few things to remember:
-
Write any catalysts/reaction conditions above the arrow
-
If more than one product is possible, write the major product (unless specified to write both). We can apply Markonikov’s rule to predict which product will be produced in greater proportion.
Need extra help with reactions of organic compounds? If you’re enrolled in Project Academy’s HSC Chemistry Course, you will get access to detailed notes written by high-scoring tutors, including more sample questions that you can use to strengthen your fundamentals!
Isomers of an Organic Compound
Isomers are compounds with the same molecular formula, but different structural formula. i.e. they contain the same number and type of atoms but are arranged differently. Isomers are important to understand in organic chemistry, especially when considering the possible structures of a compound given its molecular formula.
In HSC chemistry, we only deal with structural isomers. There are also optical isomers, but this isn’t part of the HSC syllabus so you don’t need to know about them for now. Within structural isomers, there are 3 more subtypes of isomers:
Chain Isomers
Chain isomers have a different chain parent chain length, due to branching.
- E.g. For C4H10, butane and 2-methylpropane are chain isomers, since they contain the same number of hydrogen and carbon atoms.
Position Isomers
Position isomers have the same substituents/functional groups in different positions, i.e. with different locants.
- E.g. for C3H7Cl, 1-chloropropane and 2-chloropropane are position isomers, since they contain the same number of hydrogen, chlorine, and carbon atoms.
Functional Group Isomers
Functional group isomers have the same molecular formula but different functional groups. The key ones to note are:
-
Aldehydes and ketones
- E.g. pentan-2-one and pentanal are functional group isomers.
-
Carboxylic acids and esters
- E.g. Propanoic acid and ethyl methanoate are functional group isomers.
Key Skills
Organic Nomenclature
-
Naming organic compounds
-
Flowcharts
-
Polymerisation equations
Being able to name organic compounds according to IUPAC rules is one of the most important skills in this module. Organic compounds are always named systematically with three main parts:
Prefix-main chain-suffix
E.g. 2-methylbutan-1-ol
Here are some tips on how to name organic compounds:
-
Count the number of carbons in the longest chain of the compound. This carbon chain must include the highest priority functional group.
-
We can assign a stem chain indicating the number of carbon atoms in this chain.
-
Then, assign a suffix for the highest priority functional group, which indicates what kind of compound it is.
Here is a list of suffixes for important functional groups, in order from highest to lowest priority. If your compound contains multiple functional groups, always go with the one that is the highest priority. Minimise the locant (i.e. number which defines the position) of the highest priority functional group first.
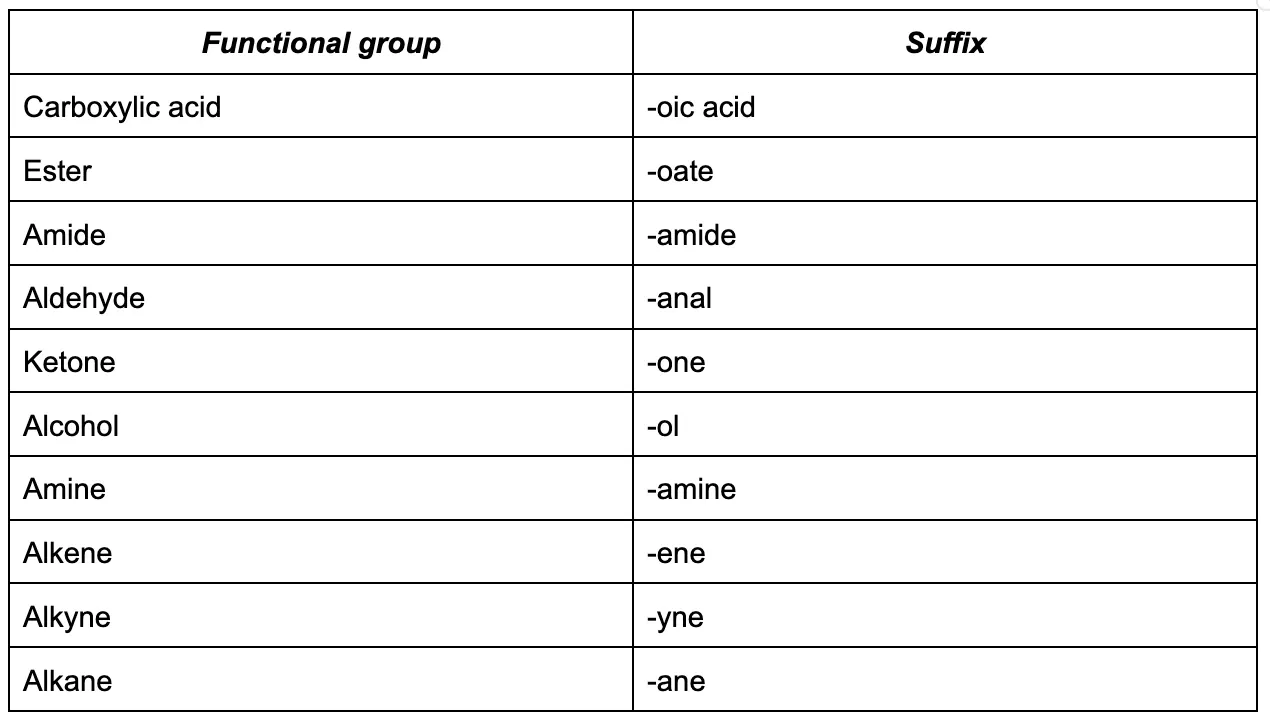


Now, we can see if there are any substituent or substituent groups on the main chain (these are groups that are connected to the parent chain of carbon atoms). These can either be a halogen or an alkyl group. These substituent groups are written in the name of the compound as a prefix. Here are some common ones:
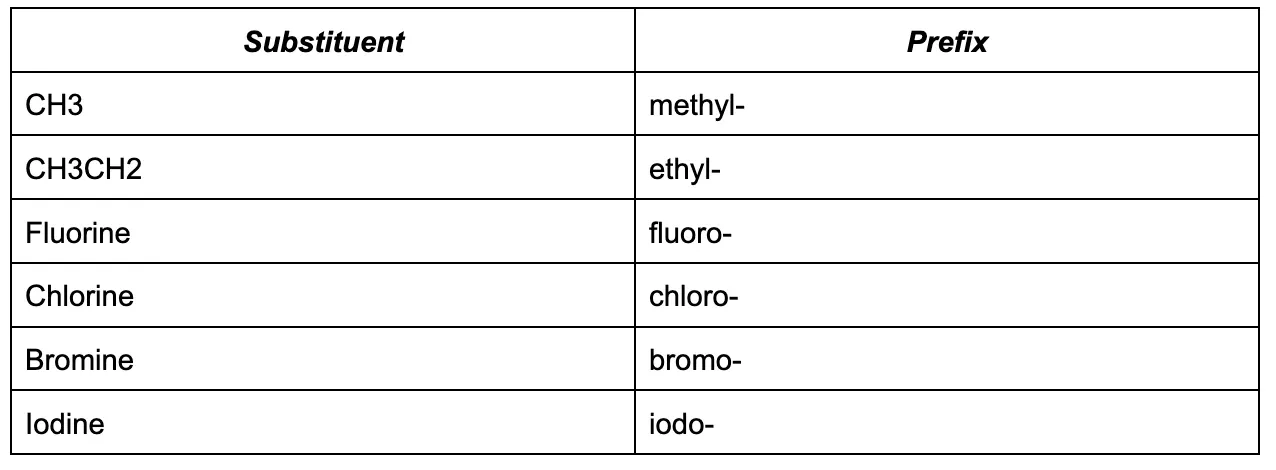


If there are multiple of the same substituent group, assign a multiplier (e.g. di-, tri-, tetra- ) to indicate how many there are. Assign each substituent group locants to show their position.
For the most part, this skill comes with practice. The more compounds you name, the faster and more accurately you would be able to do them. Make sure to ask your school teachers for practice questions, or access past papers from NESA’s official website. *Need more practice? Project Academy’s HSC Chemistry Course gives you access to a huge bank of resources on top of a weekly class, from comprehensive syllabus notes to exam-style questions that will help you master naming organic compounds. *
How to Answer Flowchart Questions
Flowchart questions are often worth many marks in HSC Chemistry exams and consolidate your knowledge of all the organic reactions studied. Here are some things to consider when drawing flowcharts:
-
Include all reaction conditions, such as catalysts, reflux, absence of UV light etc.
-
Draw full structural formulae for the reactants and products, unless indicated otherwise.
-
Remember that some reactions may yield more than one possible product!
-
Remember to use a ruler for your boxes and arrows.
How to Write Out Polymerisation Reactions
Remember that polymers are long organic molecules made up of smaller repeating sub-units, that we call monomers. Polymerisation is therefore process by which polymers are made. A key skill for HSC Chemistry Module 7, is being able to represent this process visually. There are two basic types of polymerisation that you need to be comfortable with: addition and condensation.
Addition polymerisation refers to the creation of polymer whereby monomer subunits are joined by the reaction of double bonds to form a long molecule without the loss of a small molecule (e.g. water).
Condensation polymerisation refers to the creation of a polymer through the reaction of functional groups on monomers to form a long molecule, WITH the loss of a small molecule (usually water). A common example is the formation of cellulose! The functional groups that you’ll commonly see, are alcohol + alcohol, alcohol + carboxylic acid (i.e. esterification), carboxylic acid + amine/amide.
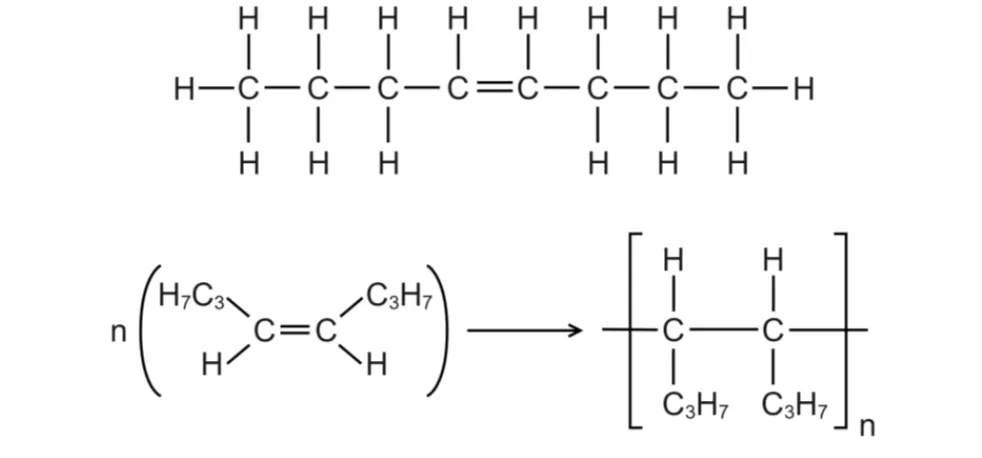
How to Write An Addition Polymerisation Reaction
-
Identify the position of the double bond.
-
Draw the carbon atoms on either side of the double bond arranged as ‘side chains’.
-
‘Open up’ the double bond to form the polymer repeating unit structure.
-
Make sure to replace the round brackets with square ones, and use “n” to represent the repeating unit structure.
Example: Draw the addition polymerisation reaction with the monomer oct-4-ene in general structural form.


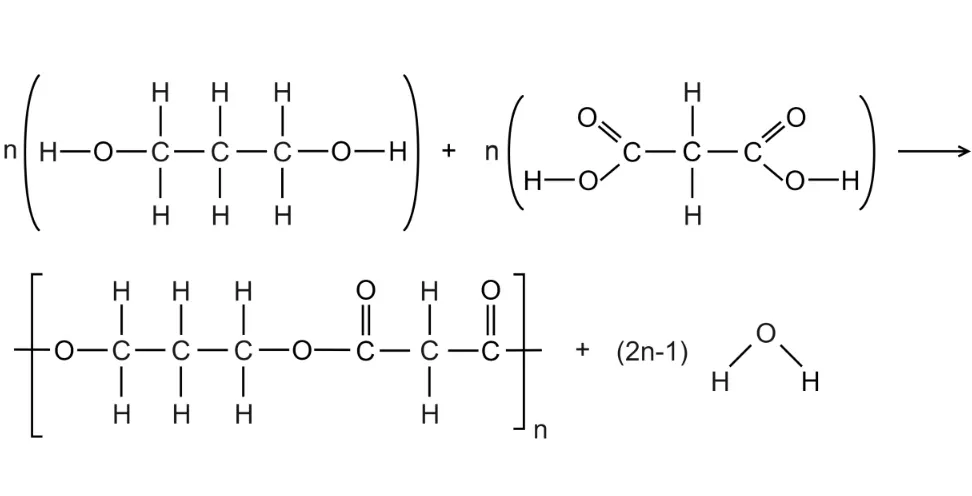
How to Write A Condensation Polymerisation Reaction
- Identify the functional groups on either side of the monomer(s) which will react.
-
Identify what small molecule will be eliminated.
-
Eliminate the atoms required from each functional group to form a bond.
-
Remember to include the small molecule that is lost.
Example: Draw the structural equation for the polymerisation reaction between propan-1,3-diol and propane-1,3-dicarboxylic acid.


Same with naming organic compounds, this skill also comes with practice. Need extra help or practice questions? If you’re enrolled in Project Academy’s HSC Chemistry Course, you will get access to heaps of sample questions and detailed notes written by high-scoring tutors, as well as ongoing feedback on your work!
Tackling Common Exam Questions
Honestly, anything can be assessed as a multiple-choice question, so we’re going to focus on themes that come up in the Extended Response section of your exam (which is worth 80 marks out of 100!).
There are several types of extended response questions. The best way to prepare is:
-
Fundamentally understand the concepts taught to you. Avoid rote learning and try and break down the cause-and-effect behind everything.
-
Structure and prepare responses to common extended response questions, and get your answers checked by a teacher or tutor.
-
Apply any feedback.
Here are some of the most common ones you could be asked to write, followed by some steps or tips to help you ace it!
-
Comparing or explaining properties of organic compounds
-
Explain what influences this property generally - e.g. how do IMFs link to boiling point or solubility? What influences reactivity and why?
-
Explain it regarding this particular organic compound, referring to what you wrote above - e.g. what IMFs does this compound have, and how does this impact boiling point?
-
-
Implications of hydrocarbons/biofuels/plastics
-
Break down your points into subcategories: environmental, economic and sociocultural implications.
-
Include plenty of chemical equations to support your response throughout!
-
If asked to make an assessment (e.g. if the commanding verb of the question was “Assess” or “Evaluate”, provide a summarising statement at the end with your conclusion.
-
-
Properties of polymers
-
To prepare for these questions I recommend drawing a table of your polymers with:
-
STRUCTURE:
-
Structural formula for the polymer
-
Any key bonds/side groups noted
-
Intermolecular forces experienced
-
-
PROPERTIES: link any properties to the corresponding structural feature above
-
Boiling/melting point
-
Tensile strength, flexibility
-
Hardness, rigidity, density
-
Stability
-
-
USES: link its uses to the properties above
- E.g. Nylon is used in clothing due to its strength and flexibility
-
-
-
The cleaning action of soaps
-
Practice drawing a diagram showing this process!
-
Ensure you include all the key steps and as much chemistry terminology as possible when describing the cleaning steps.
- E.g. The hydrophobic, non-polar hydrocarbon tail of the soap forms dispersion forces with grease whilst the hydrophilic, polar carboxylate head forms strong ion-dipole interactions with water molecules.
-
To make it easier for your marker to understand your response, you can make use of subheadings and signposting to help structure your answer.
Sample Multiple Choice Question
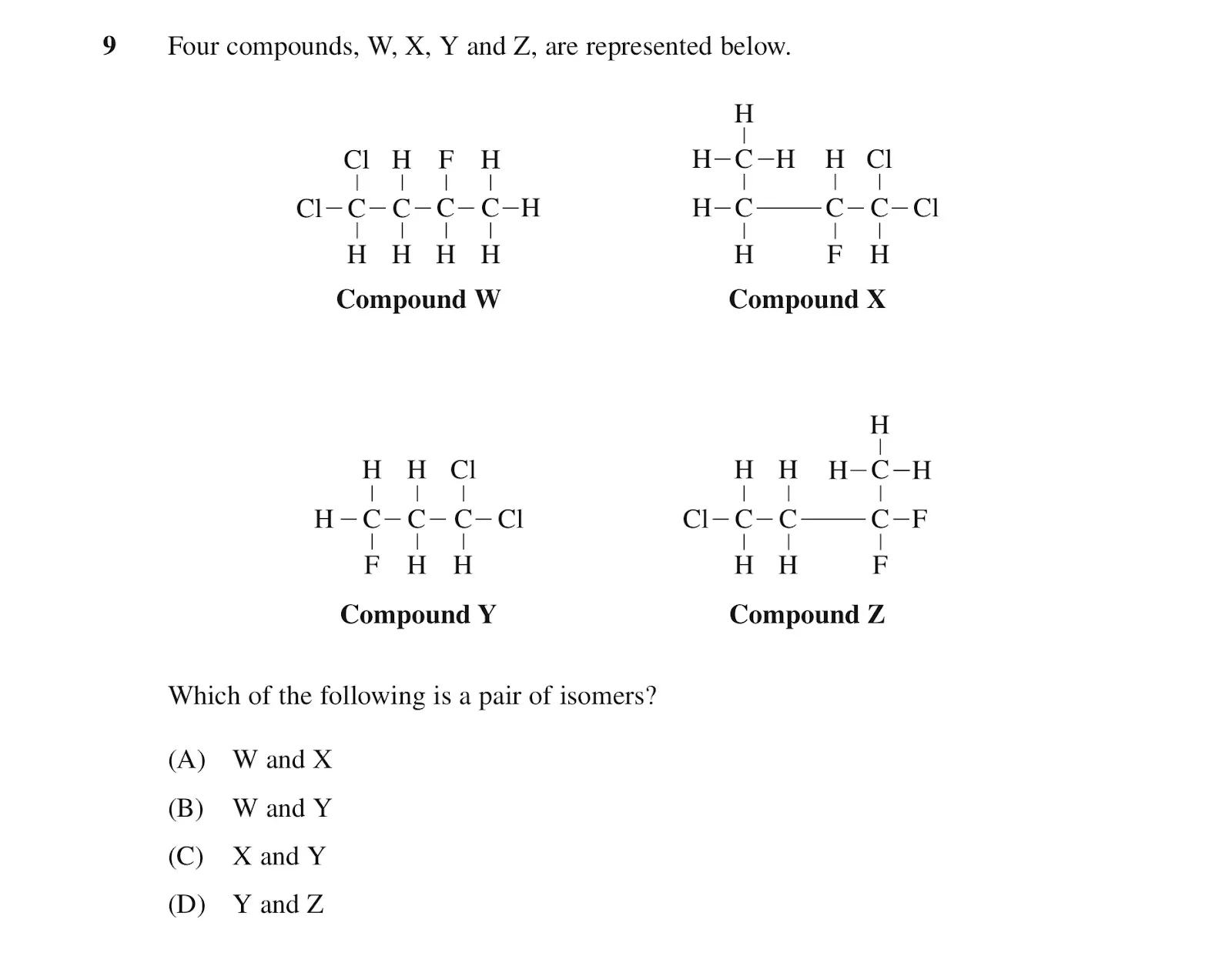
2014 HSC Q9
This is an example of an isomer question found in multiple choice. Since you should be allocating ~1.5 minutes per mark in HSC exams, it’s important to identify the fastest way to accurately solve the question.
In this case, we are asked to identify the pair of isomers within 4 compounds. Isomers are compounds with the same molecular formula but different structures. Hence we can determine the molecular formula of each compound by counting the atoms and compare to see which two compounds have identical molecular formulae.
Note that all compounds have 4 carbons, except Y which only has 3 carbons. This already eliminates option C and D since a compound with 3 carbons cannot be an isomer of a compound with 4 carbons.





Sample Short Answer Question
CSSA 2022 Trial Q32
This is a 4-mark boiling point comparison question between alcohols and alkanes. Below is an exemplar response, annotated with key features you should include in your response to get all marks possible.
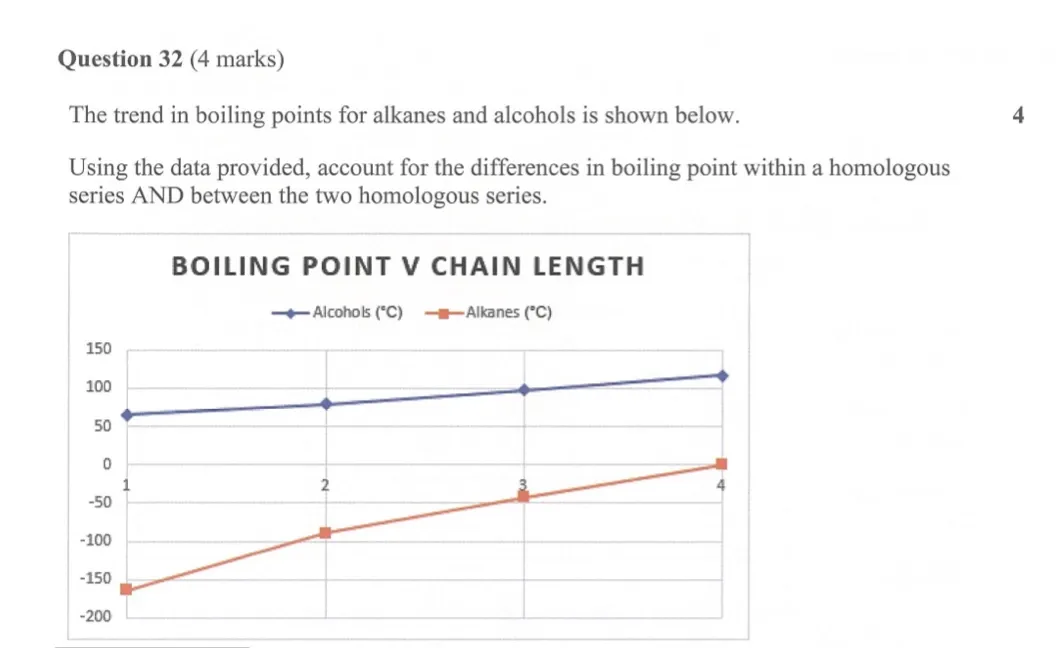





Key takeaways:
-
The marking allocation would be as follows:
-
1 mark: relating boiling point to intermolecular forces. This sets the rest of your response up.
-
1 mark: explains trend WITHIN both homologous series.
-
1 mark: describes intermolecular forces experienced by both homologous series.
-
1 mark: explains trend BETWEEN the homologous series, with reference to their intermolecular forces.
-
-
Note that the following structure was used to explain each trend:
-
A clear statement of the trend
-
Structural features of the organic compounds which contribute to this trend e.g. molecular mass, polarity, functional groups
-
An explanation in terms of intermolecular forces
-
Supporting the response with data from the graph
-
Linking back to the trend
-
-
Signposting throughout helped to structure the response and ensure nothing is missed.
Conclusion
And that’s it!
This takes us to the end of this summary article of the key concepts and skills in your study of organic chemistry! Remember to break practice drawing out organic compounds and reactions, remember their properties, signpost your responses clearly, and you’ll be fine! All the best :)
Looking for some extra help to ace HSC Chemistry? Look no further. At Project Academy, we proudly use a proven approach involving weekly masterclasses, personalised tutorials, comprehensive notes, daily support (online and offline), live pracs, and more! Find out more here.









