Light has puzzled physicists for centuries. If it puzzles you, well - you aren’t alone.
Welcome to this guide on HSC Physics Module 7 - The Nature of Light.
Unlike the preceding modules, Module 7 diverges notably, emphasising comprehension over calculations and exploring pivotal experiments. In this article, we will explore the key concepts of spectroscopy and the photoelectric effect, uncovering their significance. Additionally, we’ll delve into essential tips and tricks to study effectively, and tackle sample questions together to enhance your understanding.
Key Concepts
Spectroscopy
Understanding spectroscopy is a very important skill within the HSC physics syllabus. It’s so important that it appears in both modules 7 and 8, with very similar content between them. Without further ado, let’s jump into the main topic areas that are covered.
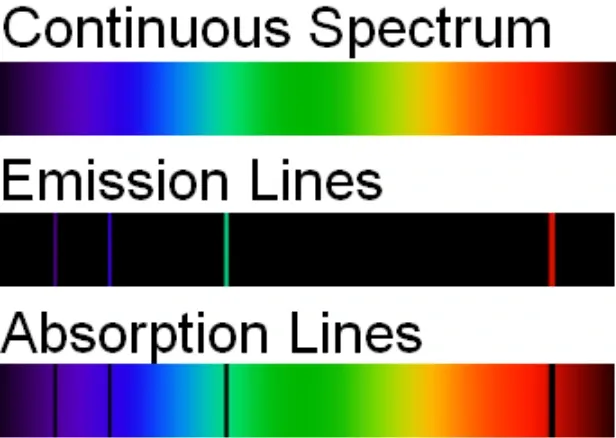
Types of spectra
There are three types of spectra that you need to know well for this course. A spectra can be viewed or observed simply by watching.
Continuous spectra
A continuous spectrum is formed when we have a perfect emitter or absorber of light. You may have heard of this as a black body, which is defined as a perfect emitter and absorber of energy. Examples of approximate black bodies include:
-
The cores of stars
-
Incandescent light bulbs
-
Very hot solids
Since black bodies emit their energy as electromagnetic radiation, this forms the rainbow spectrum that you can view by looking at reflected sunlight or reflected light form an incandescent light bulb. Make sure that you do not view the sun directly while using a spectroscope.
Emission spectra
This one requires a bit of understanding of how atoms work, and particularly the nature of energy levels. When an electron absorbs some energy, it will absorb the energy while also increasing its energy level in an atom. However, just as what comes up must come down, the electron will also return back to its original energy level. When this occurs, the energy that was originally absorbed will be released, and these specific wavelengths of light are observed as a few coloured bands on a dark background.
Furthermore, since each element has a specific electron configuration, the absorbed and emitted energies and hence wavelengths of light are different. Since these wavelengths are specific to each type of element, each element will also have a unique emission spectra.
In practice, emission spectra can be observed when passing a high voltage through a gas discharge tube.


The photoelectric effect
The latter half of the module consists of the revolutionary discoveries and theories made by the one and only Albert Einstein. Indeed, Einstein’s “miracle year” which consists of both his theories on relativity and the photoelectric effect (among other discoveries including mass-energy equivalence), are both within the latter half of the module.
Indeed, it is the photoelectric effect which won Einstein’s only Nobel Prize, albeit in 1921. It is the phenomenon responsible for solar power, and our modern understanding of light.
The wave model
The model that was believed during Einstein’s observation of the photoelectric effect was defined by Maxwell’s equations some 20-30 years earlier. Light waves were successful in explaining all the previous observations of light, including double slit diffraction and polarisation. However, it was the photoelectric effect that radically changed the model of light to what we know it as today.
The predictions of the wave model
A scientific model fails when it cannot explain new observations made through experimentation. When this occurs, we need a new, improved model that can explain this model. When tackling and understanding the importance of the photoelectric effect, it is important to see how the wave model failed at explaining certain observations that were observed within the photoelectric effect.
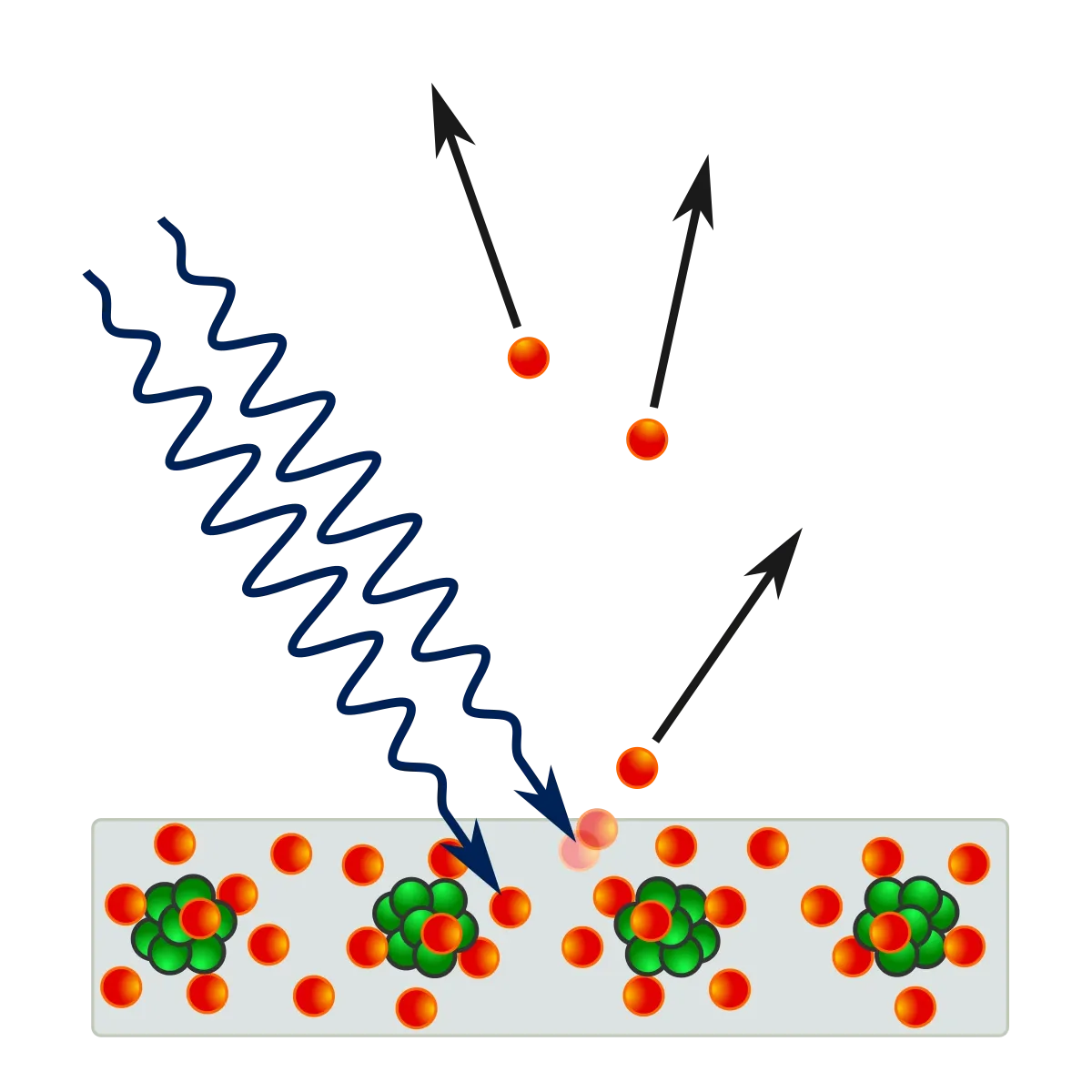
The experiment behind the photoelectric effect
As depicted in the diagram, the photoelectric effect experiment entails setting up a metal electrode exposed to monochromatic light. Upon illumination, the light carries enough energy to release photoelectrons, which migrate to the opposite electrode, generating current. The ammeter’s function is to detect this current, a result of the moving photoelectrons.


Why was this important?
When observing the photoelectric effect, the wave model of light did not match up with the observations provided. While this failed in explaining this effect, Einstein’s quantum model was able to explain these observations. The results can be summarised in the table below.
| Observation | The wave model predicted | Quantum model says |
|---|---|---|
| Existence of minimum threshold frequency for different metals. | All frequencies will still emit photoelectrons as they gain energy. | The minimum amount of energy needed to emit one photoelectron is given by the work function, and that each photon needed to overcome that work function.The frequency needed for this was called the threshold frequency. |
| Intensity has no effect on the maximum kinetic energy of emitted photoelectrons. | Higher intensity should provide a higher amount of energy to photoelectrons and hence increase . | The energy (provided by ) of every photon is used to (1) Overcome the work function and (2) Provide kinetic energy to electron. Hence if the work function is overcome the only thing that affects is the energy fo the particle. Also high intensity doesn’t mean higher amount of energy for each particular it only means more particles per unit time. |
| Instantaneous voltage/current even at low intensities. | Low intensity light requires some time to build up the energy in the photoelectrons before they are emitted. | As long as the energy (and hence frequency) of each photon is greater than the required energy to overcome the work function, it will emit a photoelectron instantaneously. |
Einstein’s Model
Einstein’s model, in essence, proposed that light consists of discrete packets of energy called photons. The intensity of light was simply the number of photons hitting a surface over a given period of time, while the frequency was the only variable affecting the energy of each individual particle. By explaining the photoelectric effect using the one-to-one principle,
The energy (E) of a photon is proportional to its frequency (f) by the equation:
Where:
-
is the energy of the photon
-
is Planck’s constant, and
-
is the frequency of the photon.
Additionally, the kinetic energy of emitted photoelectrons is given by:
Where:
-
is the maximum kinetic energy of the emitted photoelectrons,
-
is the work function of the material, the minimum amount of energy it takes to release an electron from a specific type of metal
-
is the energy of the incident photon
Since Einstein’s model was able to explain the observations of the photoelectric effect, it became a widely accepted model for light, eventually paving the way for our modern understanding of light as a wave and particle.
Key Skills
While understanding the content of module 7 is crucial to doing well in your exams, understanding how to study, revise and how to answer questions that appear commonly in your exams. Below are two tips and tricks for you used by top students.
Syllabus Secrets: Your Ultimate Guide
If you haven’t already started using the syllabus, now is the best time to start. While it may seem tricky to understand, the relevant sections of this document highlight exactly what your teachers (and the HSC) are allowed to test you. More importantly, a strong knowledge of the syllabus allows you to understand what certain questions are asking for, as well as understanding what is and is not going to be tested in your exam.
One extremely effective study technique that the syllabus allows you to do is to employ a traffic light system. This involves the following steps:
-
Get a syllabus and see which dot points you want to review.
-
Next to each dot point, mark it as red, yellow or green depending on how confident you are at this topic.
-
With the topics that are now marked as low confidence, find related short answer questions and revise the topic.
Need help revising content? Project Academy’s HSC Physics Course gives you access to some of the state’s best HSC tutors via their unlimited tutorial system. Ask any and all the questions you have!
This method allows you to effectively work on your weak points, allowing you to enter your exams confidently!
Sample Multiple Choice Question
It is said that multiple choice questions already give you all the answers. Let’s see how to approach a sample one from the 2023 HSC.



The easiest method to approach these questions is simply by and seeing which option is most correct.
However, it is first important to understand what the question is asking for. If you need to, try to highlight key words or read it twice before even glancing at the options. Some keywords here are “photoelectric effect” and “model of light subsequently proposed by Einstein.”
Next, try to unpack your knowledge about this topic, including any relevant equations. If you’ve been reading and paying attention above, you would know that Einstein’s model of light is the quantum model consisting of photons.
Some important equations may be:
Let’s see with the options:
A) This one seems valid, since if we utilise the equations and
We obtain an equation for the photoelectric effect in terms of wavelength, not frequency
Which seems to suggest that if decreases, or photoelectrons are only emitted provided that the wavelength is sufficiently small. This seems to suggest A is correct.
**However, with multiple choice questions, we should still check each other option - we may get a better option later. **
B) This is something that you will have to remember, but the intensity has no impact on maximum kinetic energy since the intensity of light does not impact the energy that each individual photon has.
C) This question tests the nature of the work function, which is different depending on which metal is used. Hence since may change in the equation:
Then may change as well.
D) This also is an observation that you need to remember. Since the emission of photoelectrons is instant, it is hence not proportional to the duration of exposure of light.
We can hence conclude the answer is A.
While not all multiple choice questions need this much working out, I recommend not rushing this section of any exams, since they are the easiest place to gain easy marks.
Sample Short Answer Question
Tackling short response questions is probably the most important skill in HSC physics exams, especially after you realise that 64 out of the 100 available marks in the 2023 HSC physics exam consisted of questions that had 2-5 available marks, which are considered short answer questions. Here are some quick tips
-
Get the key directive term. This will signal to you what you want to do in the question
-
Understand which module/s this question comes from and list related key concepts within this module
-
Get key equations
-
See if there are any calculation parts in the question.



Let’s apply our above method
-
“Show that” is a key directive term. This usually means to calculate, with our answer expected to be .
-
By seeing the word “light” and “work function” we can determine that this is a Module 7 question, particularly on the photoelectric effect.
-
Usually, to obtain key equations, I like to brain-dump a few related ones. If you can’t think of any, feel free to skim the formula sheet for any related equations that might help.
Next, we are looking to calculate maximum kinetic energy, and we are given all the values needed to calculate this value. Let’s try to do it.
(1 mark for stating the relevant equation)
Our work function is given in electron volts, but our final answer needs to be in Joules. Hence, we should first convert our work function to joules to get our final answer.
From the formula sheet:
Hence,
So we obtain, to three significant figures
(2 marks for correct conversion from eV to J)
Now, we can substitute these values into our final equation. Note that the value for Planck’s constant, h, is given to you in your data sheet. I recommend you get to understand the formula sheet well.
as required
If you want to learn more about how to answer every type of physics question, check out our other article that explains how to tackle all types of physics questions.
Conclusion
And that’s it!
In wrapping up, Module 7 of HSC Physics takes you on a journey into the fascinating world of light. As you grasp these core concepts of spectroscopy and the photoelectric effect, you’ll feel more prepared to conquer your exams. Remember to keep in mind the power of study techniques like the traffic light system and the importance of problem-solving prowess.
*Looking for some extra help to ace HSC Physics? Look no further. At Project Academy, we proudly use a proven approach involving weekly masterclasses, personalised tutorials, comprehensive notes, daily support (online and offline), and more! Find out more *here.